Chemoembolization
Chemoembolization is a minimally invasive treatment involving targeting infusion of chemotherapy directly in the artery feeding a tumor, most commonly in the liver. Chemoembolization can be used to treat primary liver cancer (Hepatocellular Carcinoma (HCC) or cholangiocarcinoma), as well as metastatic cancer which has spread to the liver from a variety of origins.
Chemoembolization is most commonly used to treat HCC as this type of cancer arises directly from the liver and often does not spread outside the liver. Chemoembolization is extremely effective at treating HCC with tumor responses greater than 70%. The procedure is considered a palliative, not curative, treatment. However, occasionally small tumors are completely killed and do not recur. Patients that develop HCC often have underlying liver cirrhosis and traditional systemic chemotherapy or external beam radiation can injure the liver as much as the tumor, which is how the chemoembolization developed.
Chemoembolization requires a small catheter, ranging from .018 to .035 inches , to be directed into liver artery under fluoroscopy (x-ray) through a small hole in the common femoral artery in the groin. After identifying the artery that is feeding the tumor(s), liquid chemotherapy or microscopic beads that elute chemotherapeutics are injected directly into the artery, which then lodge in the capillaries of the tumor. This causes a high concentration of chemotherapy in the tumor but less than 5% of the drug circulates in the blood at any given time, avoiding most symptoms associated with systemic chemotherapy.
The chemotherapeutic drug and type of beads infused into the artery is determined by the tumor type and size. This will be chosen by your doctor during your clinic visit as he/she reviews your history, imaging and labs.
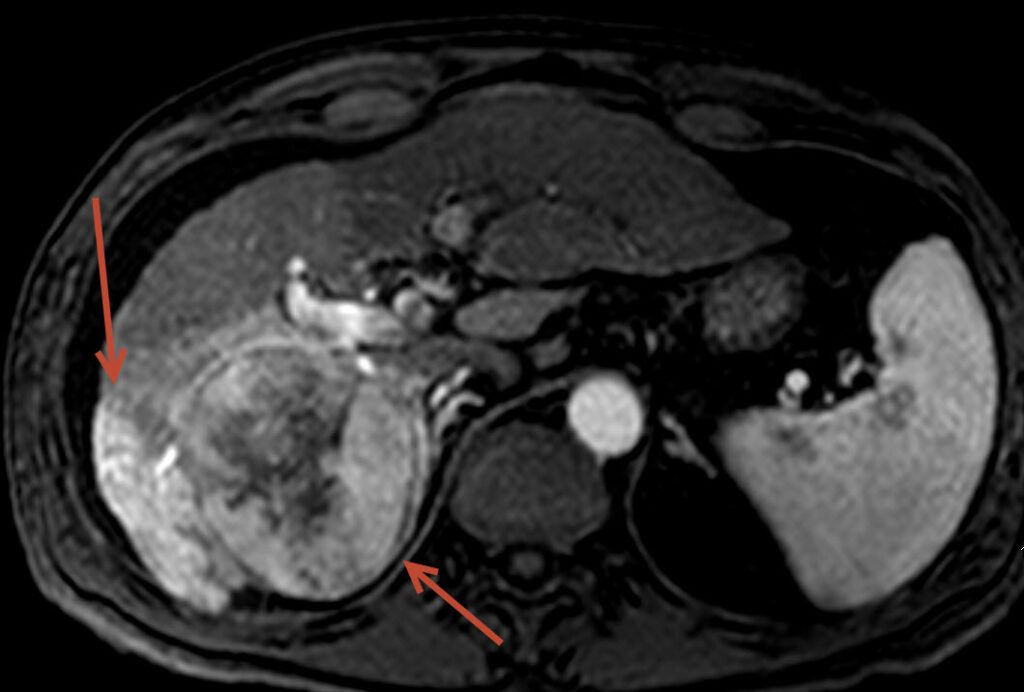
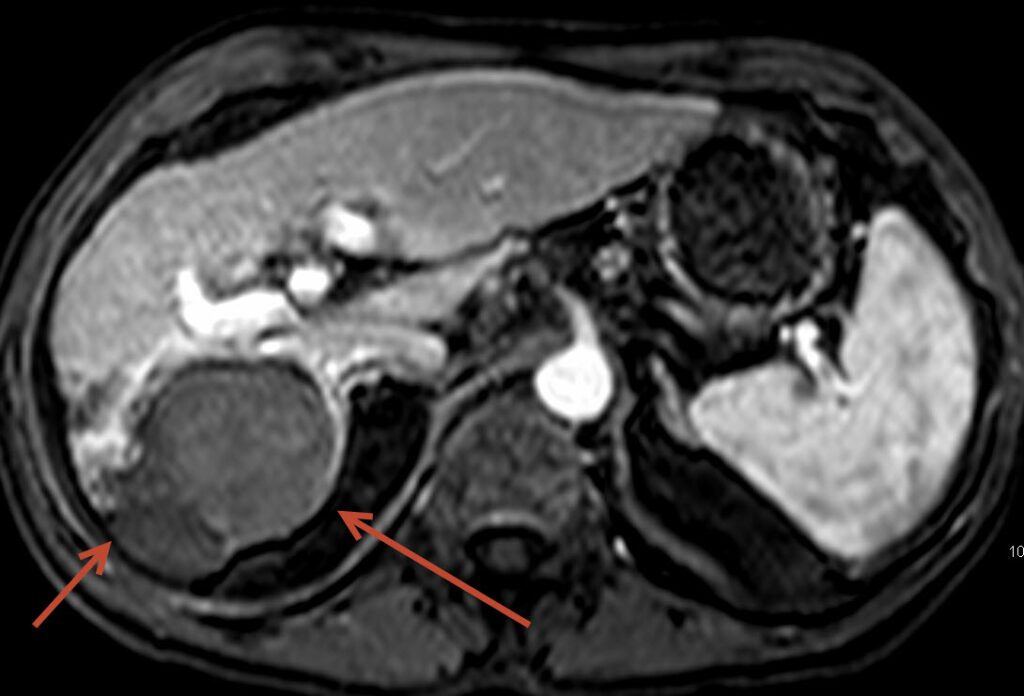
The above images show a 54 year old male treated at FIS for liver cancer, considered a non-surgical candidate with cirrhosis. The tumor filled much of the right lobe of the liver. The pretreatment images show the tumor on the left side of the screen (arrow) to be bright suggesting high blood flow, common in this type of tumor. Follow-up post chemoembolization MRI demonstrates nearly the entire tumor to be dark and necrotic (dead) without any new tumors.
Following chemoembolization, many patients have mild nausea and moderate pain for 5-10 hours and are required to stay in the hospital overnight. These symptoms are related to the tumor cell death as they are starved for blood flow. 95% of patients are discharged the morning following the chemoembolization with oral pain medication and anti-nausea medications, typically needed for 3-5 days. Many patients complain of mild fatigue for 2-3 weeks, but are able to carry on their lives normally.
Although primarily used for HCC, chemoembolization has shown promising results in patients with metastatic tumors such as neuroendocrine, colorectal, breast and esophageal cancer. Not all patients with metastatic cancer to the liver are candidates from chemoembolization, and often they may be considered for radioembolization, a similar treatment performed by FIS described on the next section under “Cancer Treatments”.
Lastly, in small to medium sized tumors, chemoembolization may be combined with radiofrequency ablation (RFA), an ablation technique used to “burn” tumors out. This technique is described under “Ablation” link in the “Cancer Treatment Section”. The combination of these two procedures has been shown to increase the likelihood of complete tumor kill and also prolong survival. If we feel a patient is a candidate for the combination treatment, it will be discussed during the initial clinic visit.
THE SERVICES LISTED ON THIS WEBSITE ARE FOR GENERAL INFORMATION PURPOSES ONLY AND DO NOT INCLUDE ALL SERVICES OF FLORIDA INTERVENTIONAL SPECIALISTS. WHILE WE STRIVE TO KEEP THE INFORMATION UP TO DATE AND CORRECT, WE MAKE NO REPRESENTATIONS OR WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, ABOUT THE CONTENT, COMPLETENESS, ACCURACY, RELIABILITY, LEGALITY, SUITABILITY OR AVAILABILITY, WITH RESPECT TO THE SERVICES CONTAINED ON THIS WEBSITE.
